Our Promise
Product Innovation Advancing
Patient Care with Excellence
Our Mission
To leverage internal expertise and external relationships to rapidly deliver high quality, low cost, high margin medical devices to the orthopedic market with a value proposition that is the right balance for the surgeon, hospital administration, OR staff, distributor, and patient.
Core Principles
- World-class scalable manufacturing
- Lean operational discipline and superior quality
- Accelerated R&D pipeline
- Novel intellectual property
- Inspire to be the industry leader
About Curiteva
Curiteva is a privately held technology and manufacturing company based in Huntsville, AL. The company was founded in 2017 in a 35,000 sq ft facility by a machinist entrepreneur, focusing on building a portfolio of proprietary spinal implants. Since its founding, Curiteva is pioneering 3D printing of porous PEEK implants with a bioactive nano-surface to revolutionize how engineered structures and implant biomaterials accelerate immunomodulation, enhance healing, and improve patient outcomes.
In the News

Renowned Neurosurgeon Dr. Kevin Foley Appointed to Curiteva’s Board of Directors

Inspire® 3D Printed Trabecular PEEK™ With HAFUSE® Lumbar Interbody System Cleared by FDA

Curiteva Announces Commercialization of its Prowess Laminoplasty System
Curiteva Featured In Additive Manufacturing Media
Curiteva® Announces FDA Clearance of Pedicle & SI Navigation Instrument System

Curiteva Announces World’s First 3D Printed PEEK Device With Regulatory Clearance Reaches Full Commercial Launch in the United States

Curiteva Strengthens Inspire® Patent Portfolio with New Patent Issued for the Novel 3D Printed Porous PEEK with HAFUSE™ Technology

First Surgeries Performed with Novel 3D Printed Porous PEEK HAFUSE Technology
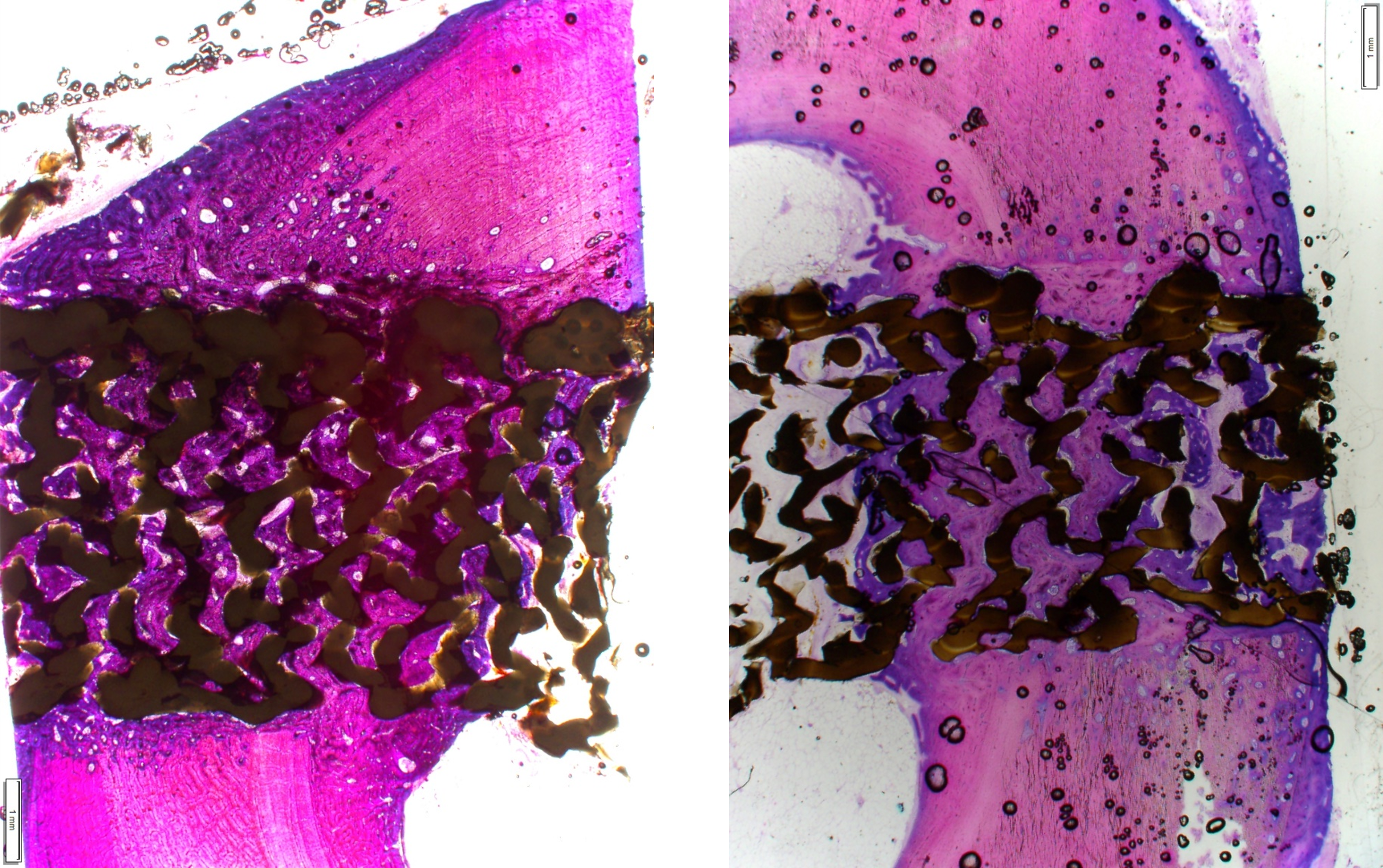
3D-Printed Porous PEEK Pioneers Reach Spine Market
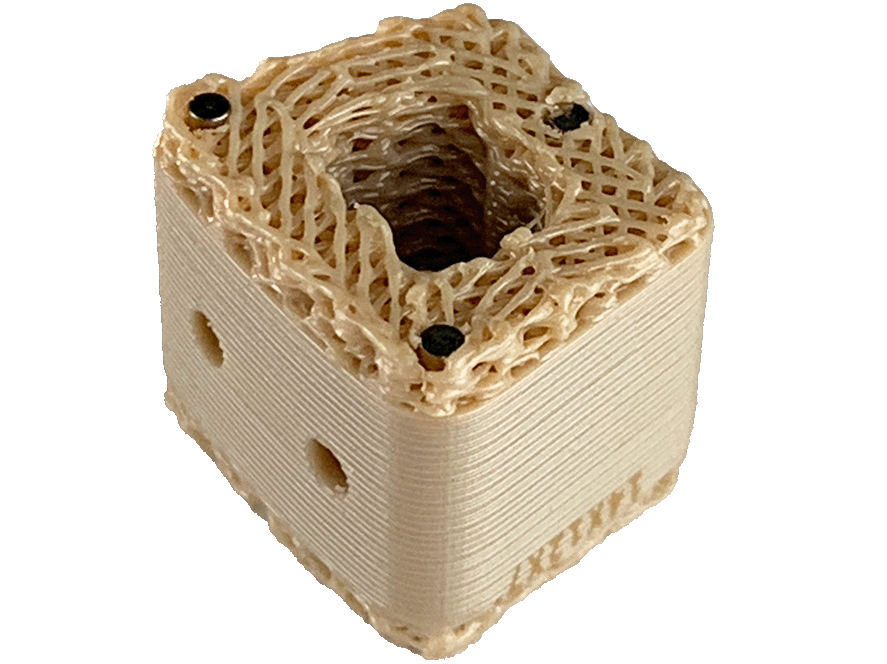












 System Features
System Features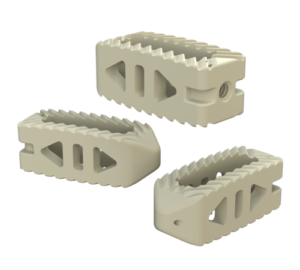
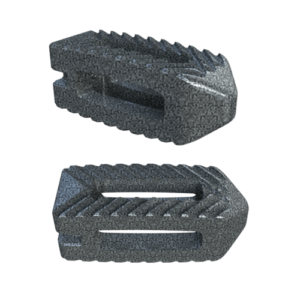
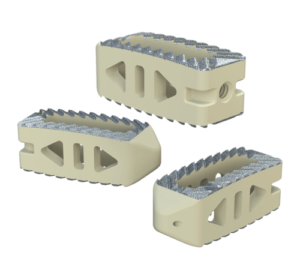


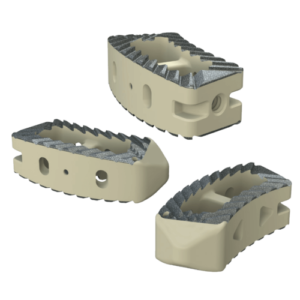



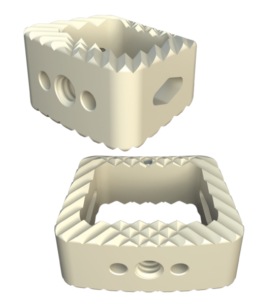 Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features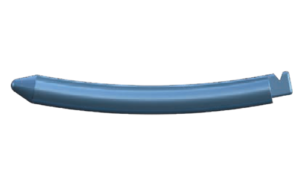 Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features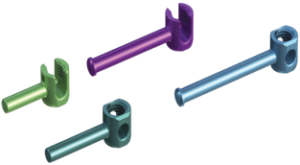 Key Features
Key Features