Todd Reith thought that 3D-printed PEEK could revolutionize the spine industry. With a background as a software and manufacturing engineer, a maker of guitars, expandable titanium implants, and IndyCar parts, he started building a printer in his home lab. After a year of tinkering, Reith achieved enough stability in the machine to print various shapes and sizes, and the confidence to talk to public medical device companies.
The initial feedback was, what’s the advantage? It was a question that Reith, then Founder and Inventor at FossiLabs, couldn’t completely answer. He returned to his lab with a mission to make a novel device that would combine the best features of PEEK and titanium. His checklist for the technology included the need to be fully porous, mimic bone and osseointegrate.
Fast forward five years to when Curiteva received FDA 510(k) clearance for Inspire Porous PEEK HAFuse Technology. The cervical interbody system is manufactured through a proprietary and patented Fused Filament Fabrication (FFF) 3D printer that is designed, programmed and built by Reith, Inventor and Vice President of Emerging Technology of Curiteva, and his colleagues.
“The leadership of the company was committed to backing a novel technology and dedicated to whatever resources were necessary to get us over the finish line,” said Reith, whose FossiLabs was acquired by Curiteva in 2020. “They were highly motivated to get this product to market.”
The Technology
Materials modifications and surface treatment techniques have been debated in spine for decades. The movement from PEEK to titanium to additively manufactured titanium implants has been a conversation of give and take regarding stiffness, strength and osseointegration. Curiteva said its studies prove that it has developed a superior product to those on the market.
“At face value, people might say, ‘Well, that’s just PEEK that they printed differently.’ That’s not the case,” said Erik Erbe, Ph.D., Chief Scientific Officer of Curiteva. “We’re able to create architectures that are stronger, tougher and have superior biologic osseointegration vs. traditional PEEK.”
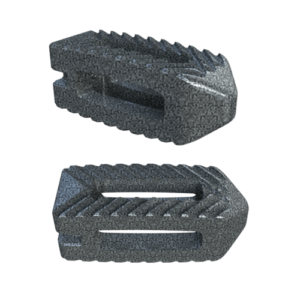
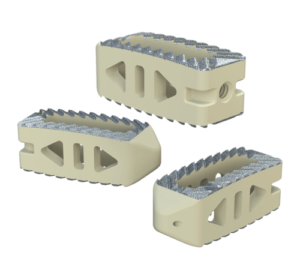
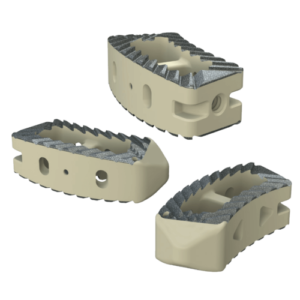
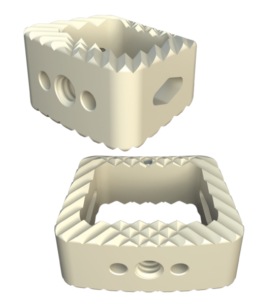
Inspire is a fully interconnected and integrated porous structure that traverses the entire implant. The technology is also the first-to-market combination of the HAFUSE nanotechnology surface and a porous PEEK structure.
The benefits and concerns of PEEK are well documented. The material’s modulus of elasticity matches that of cancellous bone, and its radiolucency allows surgeons to better assess the progress of a fusion. However, the material is hydrophobic, which can lead to fibrous tissue and prevents bone growth into the implant.
Curiteva’s 3D printing process, combined with its HAFUSE coating, maintains the benefits and overcomes the limitations of the material, according to the company.
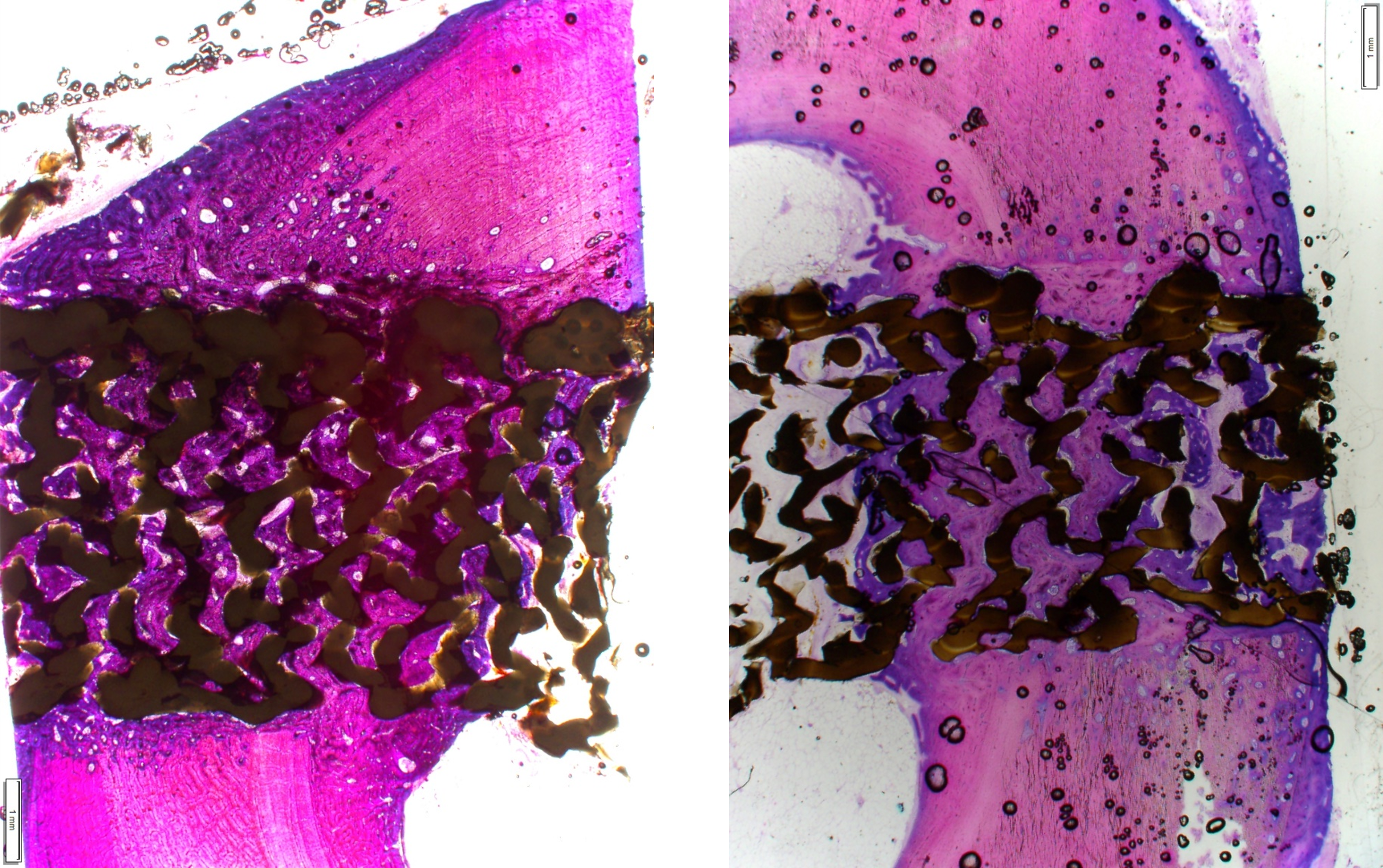
The proprietary FFF process prints diamond-shaped pores with a distribution between 100 and 600 microns. The pore shape creates superior biomechanic and biologic properties, while the pore size promotes osteoconduction, Dr. Erbe said. Printing a fully interconnected implant leads to a much higher tensile strength than compression molded PEEK. Curiteva’s studies showed that its implant’s compressive strength was 70% to 80% higher than traditional PEEK implants.
The addition of the proprietary HAFUSE Hydroxyapatite Surface Nano-Texturing is designed to promote faster and enhanced osseointegration and osteogenesis. HAFUSE, developed by Promimic, changes the implant from a hydrophobic to a hydrophilic surface. The coating is bonded to the implant surface and throughout the entire structure to enable bone to rapidly anchor directly to the implant surface and create mechanical stability.
Curiteva secured the global licensing rights to use HAFUSE on porous PEEK implants.
“We can confidently say that the implant is osteoconductive because of its interconnected pores of 100- to 600-micron pore size distribution, which is like a bone graft that we engineered into this. Because of that structure and the HA nanocoating, we have demonstrated osteogenic potential. With exposure to human bone marrow stem cells, they differentiate and express genes and proteins that are validated to promote bone formation,” Dr. Erbe said. “We have shown that the technology has osteogenic potential in cellular assays, and that it’s also demonstrated immunomodulatory response, which typically you could never say about a PEEK implant. This is why you can’t get the structure we have by any means other than our printer.”
Curiteva’s 12 week ovine histology images show osseointegration and osteogenesis.
The 3D Printer
Inspire’s technological success is directly related to the 3D printing process developed by Reith and Curiteva. While other machine companies have developed 3D printers to utilize polymers, Curiteva’s proprietary process, including its slicing and temperature, gives Inspire its enhanced mechanical and biological properties.
“Through control of the temperature, we’re able to control the amorphous state of the material, and that’s why we’re seeing these enhanced mechanical features,” Reith said. “The biggest challenge for Fused Filament printers is Z layer bonding. How do you connect the layers? You’re never at 100%. But as we’re printing, we’re generating energy to the active print layer, and we can get that bond and pull the strands simultaneously. The net result is the porous structure.”
The ability to manufacture a novel implant was not the only consideration in finalizing the printer’s design. Equipment validation and scalability were also essential to the project.
IQ, OQ and PQ are critical activities to validate manufacturing processes in medical devices. Curiteva built a dashboard that monitors the printer’s sensors and then records the information into a database that houses real-time statistical process control data.
Reith and Dr. Erbe said that FDA was highly focused on the 3D printing process, the dashboard monitoring and the implant’s final material chemistry. A typical FDA 510(k) is a 90-day process from submission to clearance. Inspire took 18 months to get it done. Curiteva partnered with Empirical Technologies, MCRA and Evonik on the project and utilized their expertise to navigate FDA’s questions and testing requirements.
“We believe we’re the pioneers in setting the standard,” Dr. Erbe said. “I think being put through the paces like that will give FDA the justification to demand that companies that come behind us have to go through the same battery of testing and the same process qualification.”
Manufacturing capabilities and regulatory hurdles aside, Curiteva needed to build an easily scalable machine. At a fraction of the required floor space and cost of a traditional CNC mill/turn center, the proprietary machines are quickly built and networked together to achieve production demands.
Curiteva placed the machines in a cleanroom that also houses post-processing. The space currently holds 10 printers and has room for 20 more.
“There are some off-the-shelf components in the machines, but all the core components are manufactured in-house,” Reith said. “We have control over the components, and we have local suppliers, such as a sheet metal company, that can punch out parts for us.”

Inspire is manufactured on Curiteva’s proprietary 3D printers, reportedly the first FFF printer to consistently print PEEK implant structures.
Next Steps
Curiteva plans to launch Inspire in key academic centers across the U.S.
The company has a 35,000-sq.-foot production facility in Huntsville, Alabama, which positions it well to control the product development and manufacturing of new implants.
While Curiteva is focused on commercializing their first interbody cage, the company is adamant that Inspire is a platform technology. They plan to build a spine portfolio with the technology, and see market potential for various implant applications, including foot and ankle, hand and wrist, and craniomaxillofacial.
“The whole mission of this venture was to develop an implant that merged the best properties of titanium and PEEK,” Reith said. “But it’s a platform technology that’s not necessarily just for spine. We’re not leveraging 3D printing because it’s the buzzword. This is a superior product.”
Reported on BONEZONE on March 23, 2023.






 System Features
System Features





 Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features