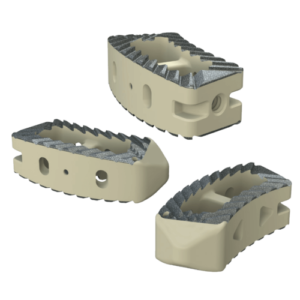
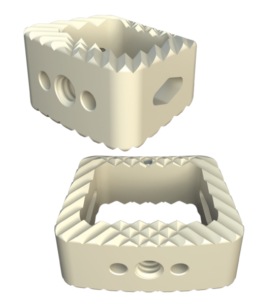
The Savant Transforaminal Lumbar (TLIF) Interbody Fusion System is offered in three different material types, multiple footprints, heights, and lordosis providing multiple options for the surgeon. The convex design offers a more accurate fit to the patient’s anatomy and offers oversized, central lateral graft windows for biologic seeding. The Savant Interbody Fusion System offers Comprehensive discectomy instruments and features instrumentation that allows for better control and more precise implant placement.
SAVANT PEEK TLIF
Key Features
- Manufactured of biocompatible PEEK polymer
- Strategically positioned tantalum radiopaque markers to optimize visibility and placement
- Angled lateral windows on anterior side of implant to retain bone graft material
- Large back surface area for increased instrument interface
- Footprints (mm): 28L x 9W, 28L x 11W, 32L x 9W and 32L x 11W
- Heights: 6 – 16mm, offered in 1mm increments
- Lordosis: 0° or 8º












 System Features
System Features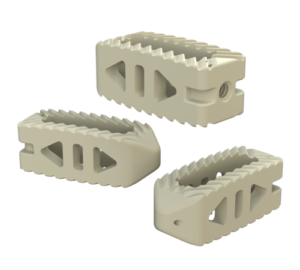
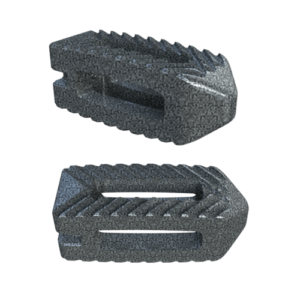
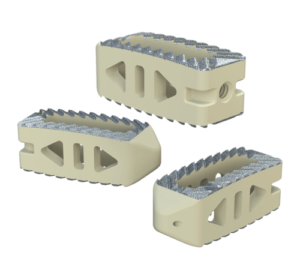





 Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features