Huntsville, AL based technology company Curiteva® announces FDA 510(k) clearance to market a Navigation Instrument System to complement their Prodigy® Pedicle Screw System and SI-LUTION® Sacroiliac Joint Fusion Systems. The pioneers in 3D printed PEEK interbody fusion technology designed these instruments to integrate seamlessly with the market leading navigation system assisting surgeons in precisely locating anatomical structures for open and MIS procedures. The system includes reusable awls, probes, taps, drills, and drivers and is avaiable for commercial release.
“Very few companies have been able to combine a highly engineered implant with user-friendly instrumentation. Curiteva’s SI-LUTION does just that and more. Now the SI-LUTION system affords surgeons the confidence and reliability of using navigation,” stated Dr. Troy Morrison, CMH Orthopedic and Spine Center.
“We are well positioned with world-class scalable manufacturing to develop instruments to further leverage a surgeons’ ability to validate implant placement, while reducing overall radiation exposure and OR time. This exciting addition to our portfolio builds on the Curiteva commitment to deliver safe, innovative, and effective products to our customers .” commented Executive Vice President, Mark Mohlman.
About Curiteva:
Curiteva is a privately held technology and manufacturing company dedicated to advancing spine surgery and improving clinical outcomes by partnering with providers and suppliers to deliver innovative and intuitive implant systems to the market. Our business is founded on a commitment to building world-class manufacturing, accelerating research and development, maintaining lean operational discipline, and delivering novel technology to meet the evolving needs of our customers and the patients they serve. For more information, please visit www.curiteva.com.

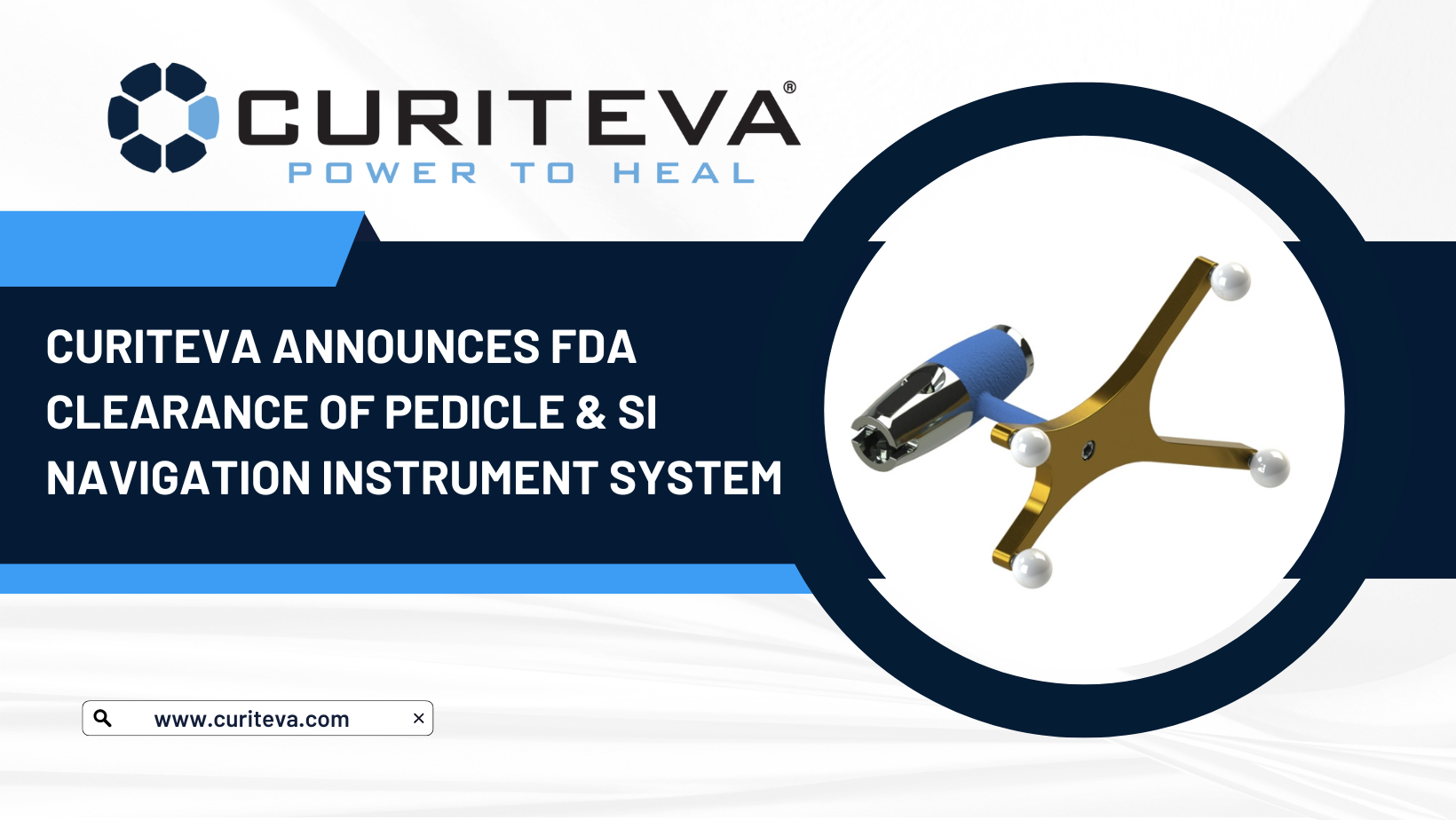




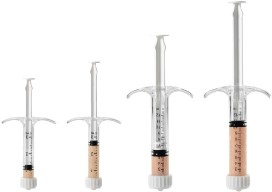
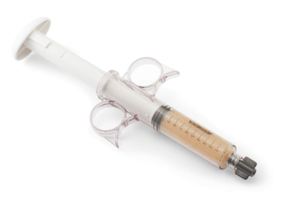

 System Features
System Features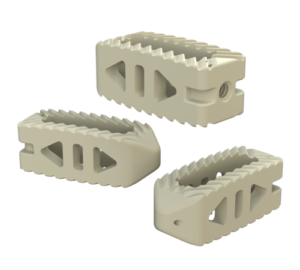
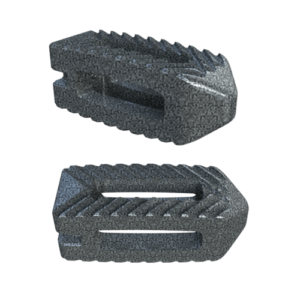
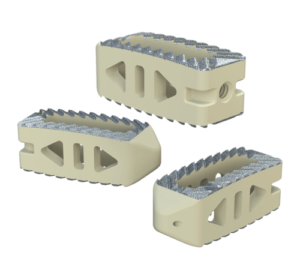


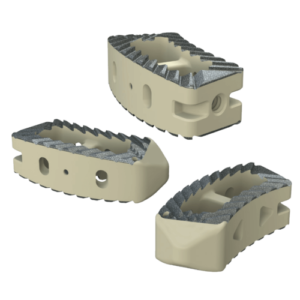



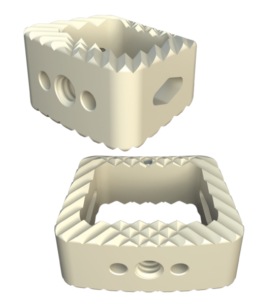 Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features