The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to a truly novel 3D printed PEEK implant.
According to the FDA 510(k) summary document, the porous PEEK cervical interbody fusion system is indicated for “intervertebral body fusion of the spine in skeletally mature patients.” It is “intended for use for anterior cervical interbody fusion in patients with cervical disc degeneration and/or cervical spinal instability, as confirmed by imaging studies (radiographs, CT, MRI), that results in radiculopathy, myelopathy, and/or pain at multiple contiguous levels from C2 – T1.” The system is “intended to be used with supplemental fixation.” Additionally, the system is “designed for use with autogenous and/or allogeneic bone graft comprised of cancellous and/or corticocancellous bone graft to facilitate fusion.”
In order to qualify for 510(k) clearance, the device must be substantially equivalent to a predicate device. The primary predicate device to this system is a cervical interbody fusion system by the same company.
Huntsville, Alabama-based technology company Curiteva, Inc. submitted the device for 510(k) clearance. OTW spoke with Curiteva Co-Founder and Chief Technology Officer Eric Linder about the system.
Linder has authored nearly 40 successful 510(k) clearances over the course of his career. He told OTW, “This is the most excited I have ever been about an FDA market clearance!”
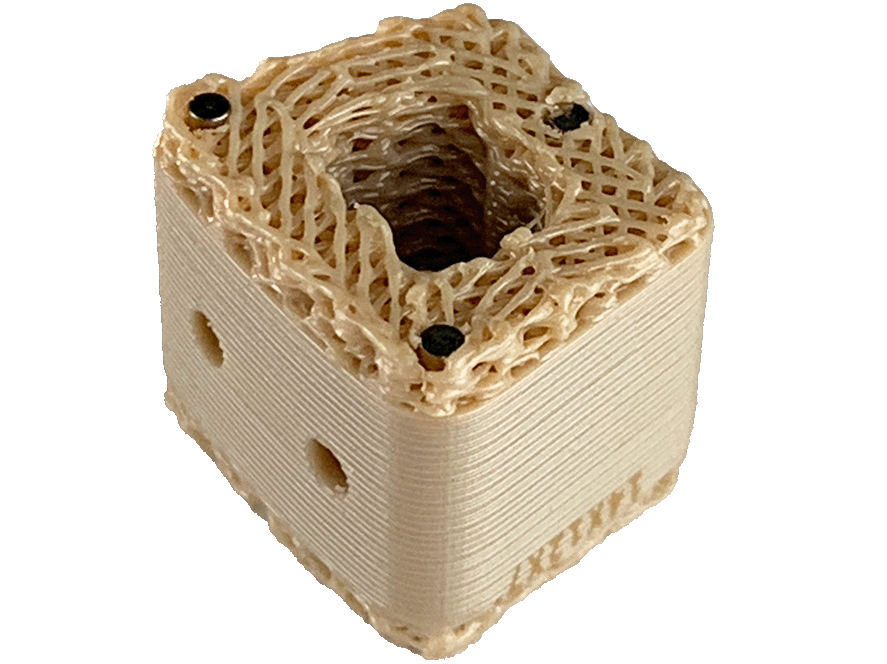
Linder also explained to OTW, “The Inspire Porous PEEK Cervical Interbody Fusion System is the first-ever 3D printed PEEK implant that the FDA has ever 510(k) cleared. This represents a massive paradigm shift of how PEEK implants may be manufactured in the future.”
Linder continued, “We designed, programmed, and built our proprietary, patented 3D printing technology from the ground up—this is a true platform technology that allows us to engineer differentiated medical devices with selective porous/dense regions using a well-known and superior implant material (e.g., radiolucency, modulus of elasticity).”
The system includes HAFUSE™ technology. According to the company’s press release, the system is manufactured with a “proprietary, patented Fused Filament Fabrication 3D-printer designed, programmed, and built by Curiteva.” This additive process is unique because it “produces a fully interconnected and integrated porous structure traversing the entire implant to promote osseointegration, improve radiographic assessment, and deliver superior biomechanics.” The combination “HAFUSE nanotechnology surface treatment and novel porous PEEK structure creates a hydrophilic, bioactive environment for cell attachment, proliferation, and healing in preclinical animal and in vitro studies.”
Reported on Orthopedics This Week on March 2, 2023.






 System Features
System Features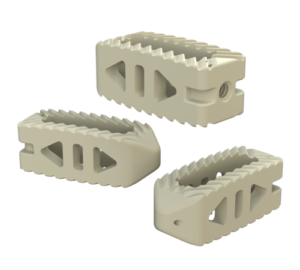
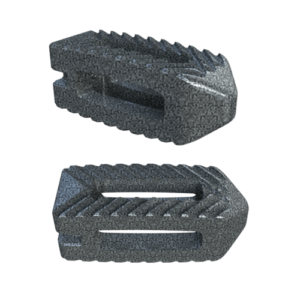
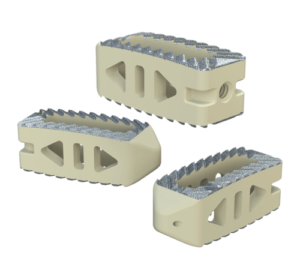


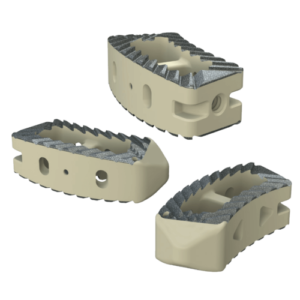



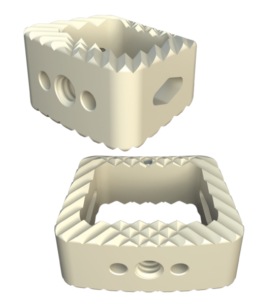 Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features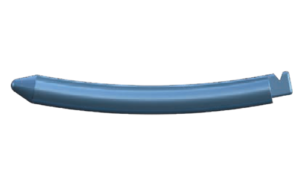 Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features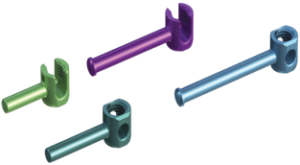 Key Features
Key Features